Searching for Blue Gold: Discovery of Marine Natural Products
Due to its rich biodiversity, the oceans can be viewed as treasure troves of interesting molecules waiting to be discovered and tapped for biotechnological application. In our laboratory, we explore a wide variety of marine organisms for their metabolites and harness them to address the growing demand for new pharmaceuticals. Furthermore, we are also looking for new approaches of expanding the chemical space of natural products derived from marine microorganisms.
Sponges and associated microorganismsSponges (Porifera) represent a significant reservoir of marine natural products, with approximately 5000 compounds identified. This accounts for approximately 30% of all marine-derived compounds that have been isolated. As sessile invertebrates, marine sponges employ small molecule compounds as a means of defense, competition, and adaptation. In the Discovery and Development of Health Products Marine Component (DDHP-MC) Phase 1 and Phase 2, we extracted 168 sponges, and 291 sponge-associated actinomycetes, resulting to 1,086 extracts. These crude extracts were all subjected to evaluation for their anticancer and antimicrobial activities. In particular, we determined the anti-inflammatory activities of monosubstituted xestoquinone analogs from the yellow sponge Neopetrosia compacta.
References: https://doi.org/10.3390/antiox11040607 https://doi.org/10.54645/MFFR36805 |
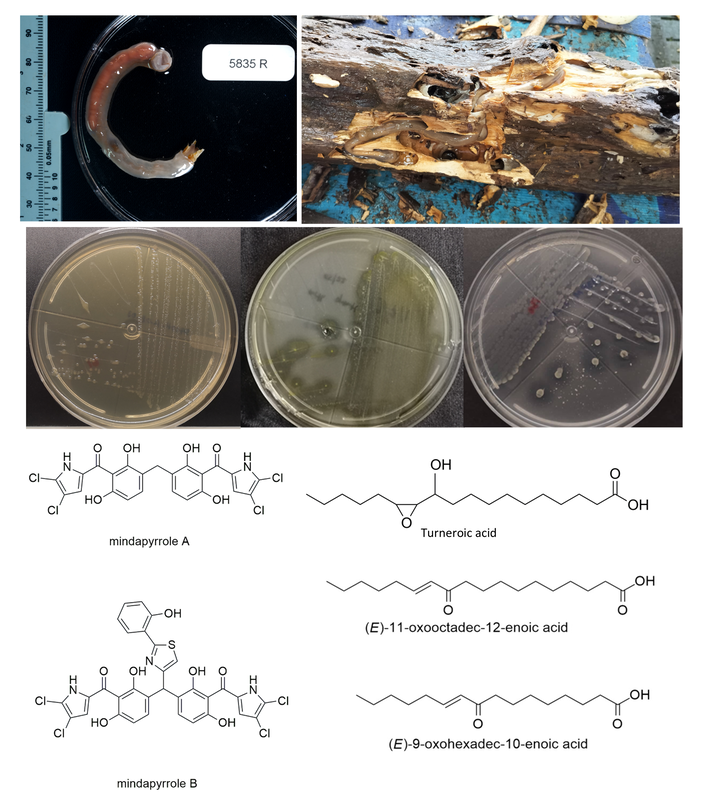
Shipworm-associated bacteriaShipworms, belonging to the family Teredinidae, exhibit a remarkable capacity for wood digestion, a function attributed to their symbiotic association with endosymbiotic bacteria. These bacteria, predominantly classified under the genus Teredinibacter, provides cellulolytic aid to degrade complex polysaccharide in wood. Aside from cellulases, Teredinibacter turnerae produce several secondary metabolites which are of particular interest due to their potential roles in defense mechanisms, signaling pathways, or as mediators of interactions within the shipworm holobiont. Initial characterization of these bioactive compounds was made possible through the research collaborations with Philippine Mollusk Symbiont Cooperative Biodiversity Group (PMS-ICBG). For instance, oxylipins capable of biofilm inhibition against S. epidermidis was described by Lacerna et al. (2020). The rich chemo-diversity in T. turnerae was further explored by Villacorta et al. (2022) through the EIDR Tamiloc Project. Aside from T. turnerae, other shipworm-associated bacteria such as P. aeruginosa were also mined for bioactive antimicrobial compounds. These secondary metabolites can be pharmacologically explored to open avenues for drug discovery and biotechnological applications.
References: https://doi.org/10.3390/md18120656 https://doi.org/10.1021/acs.jnatprod.8b00979 https://doi.org/10.3390/metabo12111152 |
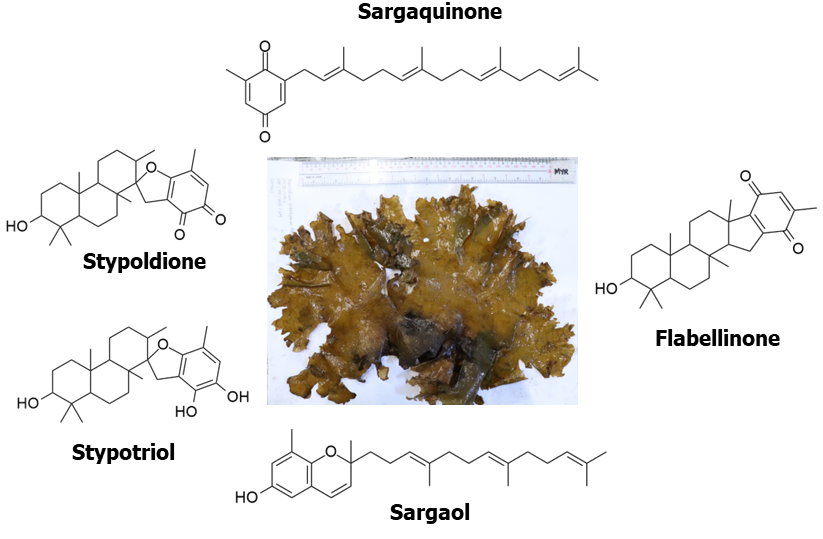
SeaweedsSeaweeds, or macroalgae, are important components of diverse marine niches. Due to their photosynthetic capabilities, many marine organisms depend on them, serving as the base of numerous trophic food chain. Their successful adaptations despite pronounced biotic pressures and lack of physical defenses suggest that seaweeds are prolific producers of noxious substances that defend them from harmful grazers. Compounds produced by seaweeds usually possess cytotoxicity and serve as a good starting point for drug discovery and development. Our CHED-funded PhycoPRO project, in collaboration with the AlgaE laboratory headed by Dr. Michael Roleda, aims to utilize these noxious substances to develop small molecules with therapeutic properties. This effort may help us understand the capabilities of macroalgae as source of therapeutic molecules and harness this unique resources for the benefit of our seaweed farmers.
|
Cone SnailsMarine cone snails (Conidae) are renowned for their remarkable diversity among marine invertebrates, boasting approximately 761 known species to date. These mollusks are famous for employing a chemically diverse array of conopeptides for capturing prey and deterring predators and competitors—a feat observed over approximately 50 million years of evolution in these slow-moving creatures. Conopeptides stand out due to their posttranslational modifications (PTMs), notably the oxidation of multiple Cys residues, which create intricate disulfide linkages. The majority of known conopeptides to date are disulfide-rich and are referred to as conotoxins, targeting a wide array of biological entities. However, disulfide-poor conopeptides, those with fewer than two disulfide bonds, have been recognized as an equally crucial component of the venom arsenal of Conoidean snails. As part of the EIDR-funded Conus Structural Exogenomics Project 1: Structural Characterization of Selected Conopeptides using Experimental Approaches, and the DOST PCHRD-funded DDHP Phase 1 and Phase 2, we purified and characterized important conopeptides from selected Conus species and tested them using our in-house assays.
References: https://doi.org/10.1002/psc.3179 https://doi.org/10.3390/toxins12080508 |
HAB-causative organisms Blooms of toxic microalgae known as harmful algal blooms (HABs) are a worldwide problem. These blooms are associated with the contamination of shellfish and other aquatic organisms with toxins that can harm the public and aquaculture industry. Among the toxins are paralytic shellfish toxins (PSTs) such as saxitoxin, neosaxtoxin and gonyautoxin which are known to be widespread and persistent throughout the Philippines. The changing oceanic conditions are bringing the emergence of new HAB-causative organisms and consequently, new classes of toxins. For example, in 2020, Karlodinium azanzae was reported for the first time in the Philippines. For this organism, toxins causing the observed toxicity has yet to be identified. As part of the PCAARRD-funded HAB Hazard Program, we identified 44-methylgambierone as the main toxin produced by three Philippine Gambierdiscus strains using mass spectrometry approaches. In collaboration with the RVA Lab, BioME Lab, and MOLab of UPMSI, we aim to identify the chemical basis of toxicity of emerging HAB-causative organisms isolated from the Philippine waters.
References: https://doi.org/10.3389/fmars.2021.767024 https://doi.org/10.3390/ijms222413332 https://doi.org/10.3390/toxins13010007 |
CyanobacteriaCyanobacteria are ancient organisms that have flourished on the planet for over 3.5 billion years. They inhabit diverse ecological niches, suggesting successful adaptation to their respective environments. Despite being simple organisms, their lack of physical defense mechanisms is compensated by the production of an arsenal of chemical cues. Many freshwater and marine cyanobacteria produce chemically diverse structures of secondary metabolites that have powerful bioactivities to fend off grazers, predators, and competitors. Structures like Dolastatin 10 serve as inspiration for the development of chemotherapeutic agents targeting cancer, inflammatory, and neurodegenerative diseases.
As part of the OVPAA-funded Balik-PhD project, various extracts from freshwater and marine cyanobacteria were screened against an array of cell-based assays for the discovery and development of anticancer, anti-inflammatory, and antimalarial agents. This project led to the identification of 12 novel secondary metabolites from our cyanobacteria extracts. References: https://doi.org/10.56899/150.05.34 https://onlineservices.ipophil.gov.ph/PatGazette/IPASJournal/V25N97_Inv_1st.pdf |
Microbial ElicitationActinomycetes and γ-proteobacteria (mainly T. turnerae) associated with shipworms and sponges are primarily considered as leading candidates for sources of new pharmaceuticals. Genomic studies reveal the presence of rich biosynthetic gene clusters with unassigned chemistry thus, highlighting the biosynthetic potential of these microorganisms to produce biomolecules with varying structural complexity and biological activities . However, many promising compounds are “silenced” or cannot be expressed under conventional laboratory conditions and thus, new approaches of coaxing secondary metabolites are explored. As part of the NAST-funded Chemodive project, we investigated the impact of microbial elicitation in the expression of cryptic secondary metabolites in T. turnerae and various marine actinomycetes.
|
Testing the Waters: Platforms for Biological Evaluation of Isolated Compounds
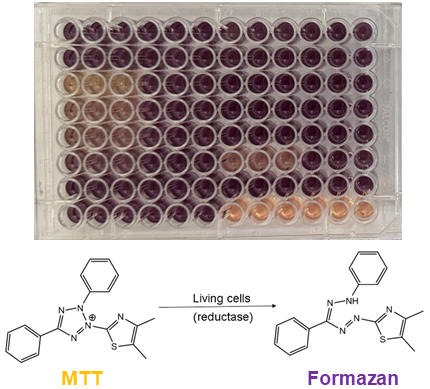
Cytotoxicity assaysCytotoxic secondary metabolites are important inspirations for the development of various anticancer therapeutics. Thus, our extracts and compounds are screened using the MTT-based colorimetric assay to identify novel structures with cytotoxic properties. The MTT-based colorimetric assay utilizes the yellow 3-(4,5-dimethylthtiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), which enzymatically converts to purple formazan upon contact with cellular oxidoreductases in living cells. Cancer cells typically utilized in our lab include HCT116 colon cancer cells, and MCF-7 human breast cancer cells.
|
Dorsal Root Ganglion assayIntracellular calcium plays a vital role in the release of neurotransmitters, cellular excitability, and the overall synaptic activity of neurons. Dysregulation and dysfunction of neuronal calcium levels are common occurrences in neurological disorders. To explore this concept, we employ a high-throughput phenotypic screening method to examine disturbances in intracellular calcium caused by active compounds or crude extracts when applied to diverse neurons found in the dorsal root ganglion (DRG). These electrically excitable soma cells within the DRG offer access to a variety of functional ion channels, G protein-coupled receptors (GPCRs), and other surface proteins that contribute to the DRG's capacity to transmit different sensory experiences such as touch, pain, temperature, itch, and more. The activity within DRG neurons has been associated with advancements in anti-pain research and therapeutic interventions.
|
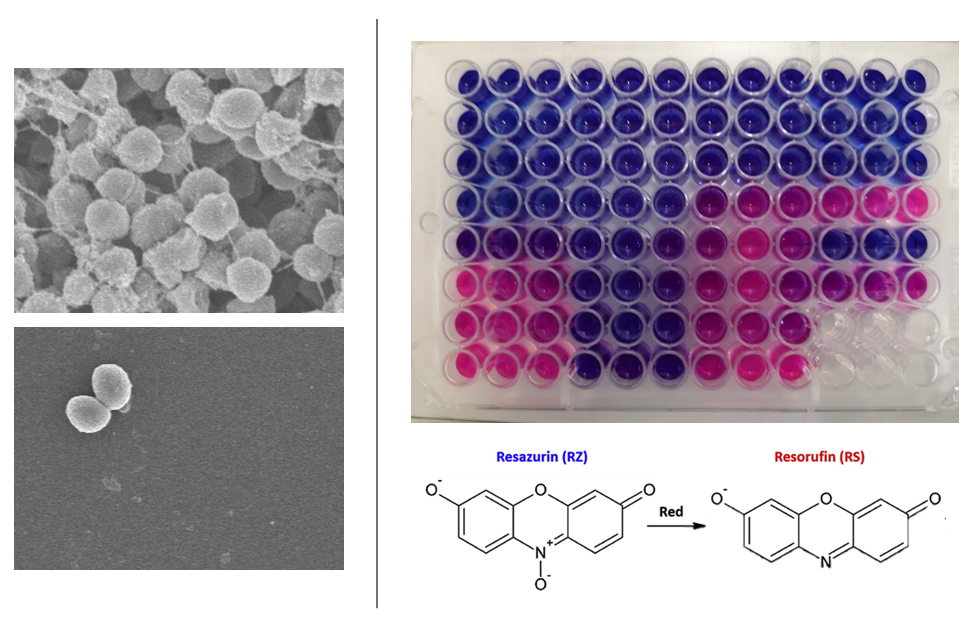
Antimicrobial assaysUncover the antimicrobial potential of your crude extracts and pure compounds against the threats of ESKAPE pathogens. Our resazurin-based microdilution assays provide antimicrobial quantification for the following pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter aerogenes. We can also analyze the efficacy of your samples in preventing Staphylococcal biofilm formation.
|
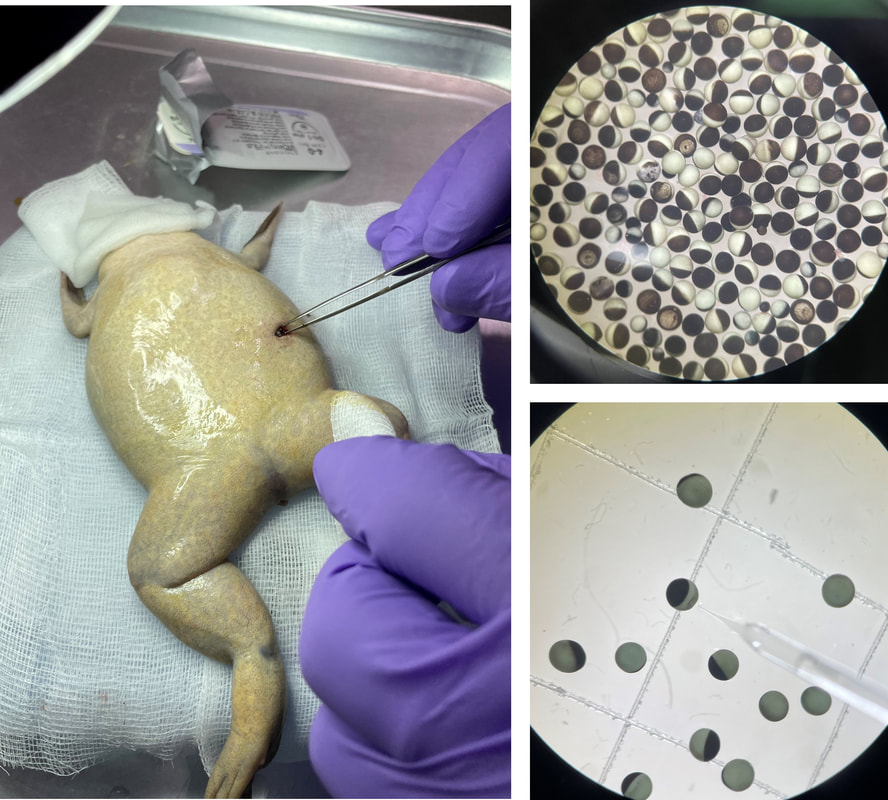
Electrophysiology AssaysIon channel activity changes as channels briefly open and close to carry out specific cellular functions. This process induces rapid adjustments in membrane potential or action potential, measured experimentally and electrically using microelectrodes and amplifiers. Measurement involves voltage clamping, with the simplest method being the two-electrode voltage (TEV) clamp. The receptor of interest is expressed in Xenopus (African clawed frog) oocytes, which, due to their large size and low expression of endogenous receptors, allow exogenous expression of investigated ion channels and receptors. Current is assessed from inside to outside the cell, where outward or positive currents signify positively charged ions exiting the cell (e.g., K+ ions during repolarization), and inward or negative currents indicate positively charged ions entering the cell (e.g., Na+ ions during depolarization or nAChR opening).
|
Decoding the Blueprint: Identification of Putative Biosynthetic Gene Clusters of Marine Natural Products
Advances in sequencing technology have revolutionized the field of natural product discovery by facilitating the identification of biosynthetic gene clusters (BGCs) responsible for the production of these compounds in microorganisms. The identification of putative biosynthetic gene clusters involves searching for specific genetic signatures, such as conserved sequences or motifs, that are indicative of genes involved in the synthesis of bioactive molecules. As part of our ongoing efforts to identify bioactive molecules from marine invertebrate-associated microorganisms, we also aim to determine the BGCs responsible for the production of these compounds. We also integrate genomics data to mass spectrometry-based metabolomics data to identify potentially new classes of compounds produced by priority microorganisms.
Reference: https://doi.org/10.54645/MFFR36805
Reference: https://doi.org/10.54645/MFFR36805
Addressing the Supply Issue: Using Ecology-Driven Approaches to Increase Production of Marine Natural Products
Despite the abundance of bioactive natural products derived from sponges, the development of these compounds has faced challenges due to the persistent "supply problem," characterized by the difficulty in obtaining a consistent and sufficient supply. Sponges typically yield small quantities of bioactive compounds, and large-scale collection of sponge tissues to address the supply issue is unsustainable. Various approaches have been suggested to tackle the supply problem, including chemical synthesis, sponge cell culture, recombinant expression, and mariculture. Chemical synthesis, often the initial method attempted, may not always be effective due to the intricate structures of natural products. Mariculture of sponges emerges as a promising solution to the supply problem, given the rapid regeneration capabilities of sponges. Mariculture and transplantation studies have been conducted on numerous sponge species with industrial and medicinal significance, aiming to enhance bulk sponge biomass and/or the production of specific bioactive secondary metabolites. These studies seek to comprehend the impact of biotic factors (e.g., life cycle, symbiont presence, predator pressure) and abiotic factors (e.g., water temperature, depth environment) on metabolite production, ultimately allowing for the formulation of an optimal cultivation strategy. In collaboration with the IMBIBE Lab of UPMSI, under the DOST PCHRD-funded DDHP Program Phase 1 and Phase 2, we looked into the mariculture potential of the renieramycin-producing blue sponge Xestospongia sp.
References:
https://doi.org/10.1016/j.aquaculture.2018.12.059
https://doi.org/10.1002/aff2.98
References:
https://doi.org/10.1016/j.aquaculture.2018.12.059
https://doi.org/10.1002/aff2.98